这是演示店铺,请务下单付款,避免造成你的财物损失。
liposomes
PRODUCT DESCRIPTION
The phospholipid bilayers are composed of phosphatidylcholine and cholesterol. All liposomal formulations are suspended in sterile phosphate buffered saline (PBS):
– 10 mM Na2HPO4;
– 10 mM NaH2PO4;
– 140 mM NaCl.
All our liposomes are slightly negatively charged and will therefore not clog together. The liposomes in the suspension are not sized, meaning that it contains smaller and larger liposomes as well (up to 3 micron). On average, the size of the liposomes are 1.7 micron.
Clodronate liposomes: a suspension of artificially prepared lipid vesicles encapsulating clodronate. The concentration of clodronate in the suspension is ca. 5 mg / mL. Clodronate is encapsulated in the liposomal vesicles in the form of CH2Na2Cl2O6P2 · 4 H2O.
PBS liposomes: a suspension of artificially prepared lipid vesicles encapsulating an aqueous PBS solution. These do not contain clodronate and can be used for control experiments.
Fluorescent DiI Liposomes: a suspension of artificially prepared lipid vesicles encapsulating an aqueous PBS solution, labelled with the fluorochrome DiI. These do not contain clodronate and can be used to investigate whether liposomes injected via a particular administration route are able to reach the macrophages to be studied.
STORAGE AND DIRECTIONS OF USE
Upon arrival, liposomes should be stored between 4 – 8 ºC (or 39 – 47 ºF). The liposomal suspensions should never be frozen, nor be exposed to extreme high temperatures. This can cause disturbances to the phospholipid bilayers, possibly leading to leakage of clodronate out of the liposome.
Before administration, let the liposomes reach room temperature first and gently shake or stir the suspension. Liposomes tend to precipitate after some time, causing an inhomogeneous distribution in the vial. When injection takes too much time, the liposomes may even precipitate in the syringe. If multiple animals are injected using the same syringe, this can cause differential dosing.
Dilution of the suspension is discouraged, but if necessary use PBS or saline.
We advise our customers to use our liposomal formulation within 16 weeks after shipment. Use after the expiry date has occurred is strongly discouraged. After this period the risk of contamination increases, and a slight loss of function could occur.
Macrophages play an important role in immune and non-immune defence mechanisms. They form a first line of defence against bacterial, viral and other forms of microbiological contamination penetrating into the bodies of vertebrates. Macrophages are large cells, found in almost all bodily tissues where they can have varying forms and names (e.g. Kupffer cells, alveolar macrophages, microglia, osteoclasts, red pulp macrophages). Macrophages “scavenge”, they ingest and digest all foreign substances, microbes, cancer cells and cellular debris that might be potential pathogens. This process is called phagocytosis. Macrophages further regulate functions of many non-phagocytic cells, mainly through mediation of soluble molecules such as cytokines and chemokines. They are involved in innate immunity, adaptive immunity and can have (anti-) inflammatory effects.
Liposomes are artificially prepared spheres and consist of concentric phospholipid bilayers. When phospholipids are dispersed in water, the hydrophilic heads will make up both outer parts of the liposome, whereas the hydrophobic fatty acid groups will make up the inner part (see figure 1).
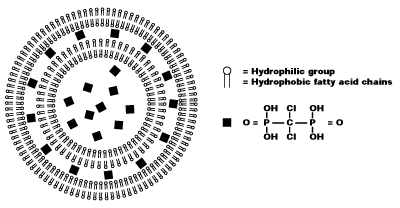
Aqueous compartments separate the bilayers, and hydrophilic molecules can be dissolved in it, resulting in liposome-encapsulated molecules. Clodronate (dichloromethylene-bisphosphonate or Cl2MBP) is a hydrophilic molecule that can be encapsulated within phospholipid bilayers. Free clodronate does not easily cross cell membranes, and is rapidly cleared (i.e. within minutes) from circulation by the renal system. However, when entrapped in a liposome, the clodronate liposome is ingested by macrophages and cannot escape it (see figure 2).

The phospholipid bilayers are digested by lysosomal phospholipases, whereas clodronate is not digested and remains in the macrophage. The more phospholipid bilayers and liposomes are ingested by the macrophage, the more clodronate will accumulate within the macrophage. Exceeding a certain intracellular concentration, clodronate will eliminate the macrophage by initiating its programmed cell death, i.e. apoptosis.
Administration protocols
Administration protocols should be chosen carefully and depend on several factors, for instance: the type of macrophage you intend to deplete (e.g. Kupffer cells, alveolar macrophages, microglia, osteoclasts, red pulp macrophages), the time in which you intend to maintain depletion (e.g. short or long term), the (animal) model, and other experimental factors. In vitro application of clodronate liposomes is possible, albeit that they are specifically suitable to study macrophages in vivo. Below you can see schematic representations of the tissues and macrophages that can be reached through different administration routes. Please note that not all tissues and routes are represented here.
Abbreviations: AL = lung alveoli, BM = bone marrow, BR = brain, BV = blood vessels / circulation, DA = draining area of lymph node, EY = eye, GU = gut / intestines, IV = intravenous delivery, KI = kidney, LI = liver, LN = lymph node, LU = lung, LV = lymph vessels, PE = peritoneal cavity, SP = spleen, SY = synovial cavity in joint, TE = testis, TR = trachea.
In general, it is recommend to inject 100 µL of suspension / 10 grams of animal weight for intravenous injection. Raising the dosage considerably may lead to blockage of capillaries. Intravenous administration of clodronate liposomes will lead to maximum depletion of liver and spleen macrophages in ca. 24 hours. Dependent on the subset of macrophages they will remain depleted for ca. 5 days. After that time, new macrophages will replace the depleted ones: macrophage precursors, monocytes, that are formed in bone marrow and released in circulation will arrive at their destination and further differentiate into mature macrophages. Monocytes, macrophage precursors, can also be depleted. To prevent macrophage repopulation, multiple injections can be administered every 2-3 days to target monocytes. See for instance: Sunderkötter, C., Nikolic, T., Dillon, M. J., Van Rooijen, N., Stehling, M., Drevets, D. A., & Leenen, P. J. (2004). Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. The Journal of Immunology, 172(7), 4410-4417.For intraperitoneal administration, the recommended injection dosis (i.e. 100 µL of suspension / 10 grams of animal weight) can be increased considerably. This route also depletes peritoneal macrophages, but will take longer to deplete macrophages in liver and spleen (ca. 3 days). Depletion is slower and more gradual, since the liposomes have to be carried from the peritoneal cavity to circulation by lymph flow via the thoracic duct, which is a passive form of transport.
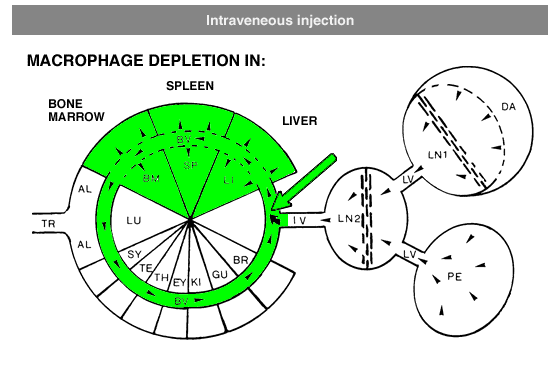
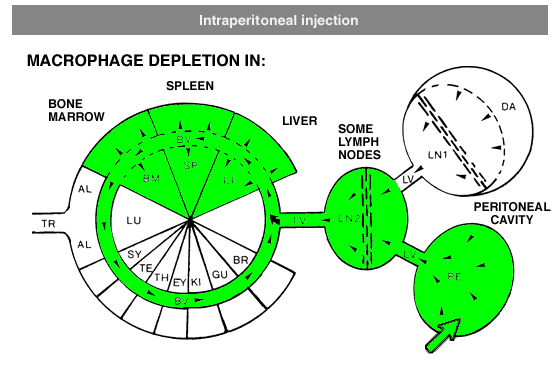
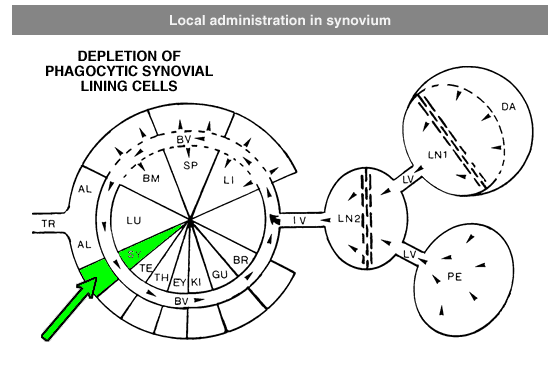
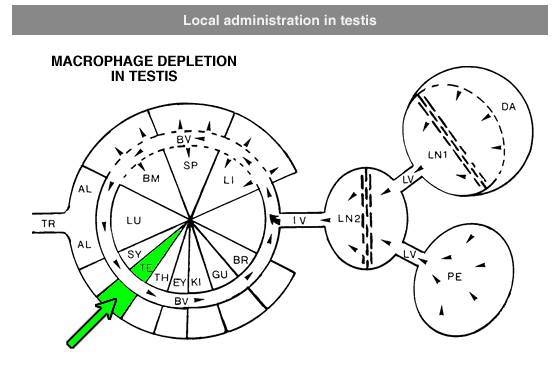

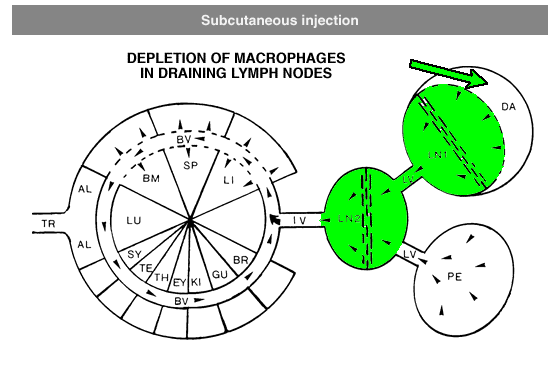
Local administration is often required to target macrophages that are difficult to reach, such as macrophages in testis or the phagocytic synovial lining cells.For depletion of alveolar macrophages, clodronate liposomes can be administered intranasally as well as intratracheally. The difference is that intranasal liposomes may be spoiled in the oesophagus if not administered properly, whereas intratracheal instillation will deliver all liposomes in the lung. Please note that these routes only target alveolar macrophages, and not interstitial macrophages. These can be targeted through depletion of blood monocytes in circulation. See for instance: Huang, L., Nazarova, E. V., Tan, S., Liu, Y., & Russell, D. G. (2018). Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. Journal of Experimental Medicine, 215(4), 1135-1152.For subcutaneous injection the maximum volume to be injected depends on the storage capacity of the injection site.
RESULTS
Sections of mouse liver, stained with monoclonal antibody F4/80 Normal liver with positive Kupffer cells
Liver of a mouse, 2 days after injection with clodronate-liposomes. Kupffer cells have been depleted

Sections of mouse spleen stained for acid phosphatase Normal spleen with positive splenic macrophages

Spleen of a mouse 2 days after injection with clodronate liposomes. Only few macrophages are left

REFERENCES
- First publication of the approach
Van Rooijen, N., & Van Nieuwmegen, R. (1984). Elimination of phagocytic cells in the spleen after intravenous injection of liposome-encapsulated dichloromethylene diphosphonate. Cell and tissue research, 238(2), 355-358.
- Application in research and therapeutics
van Rooijen, N., & van Kesteren-Hendrikx, E. (2002). Clodronate liposomes: perspectives in research and therapeutics. Journal of liposome research, 12(1-2), 81-94.
- Elimination and blocking of macrophages in general
Rooijen and Annemarie Sanders, N. (1997). Elimination, blocking, and activation of macrophages: three of a kind?. Journal of leukocyte biology, 62(6), 702-709.
- Mechanism macrophage apoptosis mediated by clodronate liposome
Van Rooijen, N., Sanders, A., & van den Berg, T. K. (1996). Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. Journal of immunological methods, 193(1), 93-99.
- Depletion of Kupffer cells in liver
Van Rooijen, N. I. C. O., & Sanders, A. (1996). Kupffer cell depletion by liposome‐delivered drugs: comparative activity of intracellular clodronate, propamidine, and ethylenediaminetetraacetic acid. Hepatology, 23(5), 1239-1243.
- Depletion of macrophages in spleen
Van Rooijen, N., Van Nieuwmegen, R., & Kamperdijk, E. W. A. (1985). Elimination of phagocytic cells in the spleen after intravenous injection of liposomeencapsulated dichloromethylene diphosphonate. Virchows Archiv B, 49(1), 375.
Van Rooijen, N., Kors, N., & Kraal, G. (1989). Macrophage subset repopulation in the spleen: differential kinetics after liposome‐mediated elimination. Journal of leukocyte biology, 45(2), 97-104.
Van Rooijen, N., Kors, N., Vd Ende, M., & Dijkstra, C. D. (1990). Depletion and repopulation of macrophages in spleen and liver of rat after intravenous treatment with liposome-encapsulated dichloromethylene diphosphonate. Cell and tissue research, 260(2), 215-222.
- Depletion of macrophages in lymph nodes
Delemarre, F. G. A., Kors, N., Kraal, G., & Van Rooijen, N. (1990). Repopulation of macrophages in popliteal lymph nodes of mice after liposome‐mediated depletion. Journal of leukocyte biology, 47(3), 251-257.
- Depletion of alveolar macrophages in lung
Thepen, T., Van Rooijen, N., & Kraal, G. (1989). Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. Journal of Experimental Medicine, 170(2), 499-509.
- Depletion of peritoneal macrophages
Biewenga, J., van der Ende, M. B., Krist, L. F., Borst, A., Ghufron, M., & van Rooijen, N. (1995). Macrophage depletion in the rat after intraperitoneal administration of liposome-encapsulated clodronate: depletion kinetics and accelerated repopulation of peritoneal and omental macrophages by administration of Freund’s adjuvant. Cell and tissue research, 280(1), 189-196.
- Depletion of testis macrophages
Bergh, A., Damber, J. E., & Van Rooijen, N. (1993). Liposome-mediated macrophage depletion: an experimental approach to study the role of testicular macrophages in the rat. Journal of endocrinology, 136(3), 407-NP.
- Depletion of phagocytic synovial lining cells in joints
Blom, A. B., van Lent, P. L., Holthuysen, A. E., van der Kraan, P. M., Roth, J., van Rooijen, N., & van den Berg, W. B. (2004). Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthritis and cartilage, 12(8), 627-635.
- Depletion of perivascular and meningeal macrophages in the central nervous system
Polfliet, M. M., Goede, P. H., van Kesteren-Hendrikx, E. M., van Rooijen, N., Dijkstra, C. D., & van den Berg, T. K. (2001). A method for the selective depletion of perivascular and meningeal macrophages in the central nervous system. Journal of neuroimmunology, 116(2), 188-195.
 首页
首页 400-620-6333
400-620-6333



 危险品化学品经营许可证(带存储)
危险品化学品经营许可证(带存储)