计算溶液所需的质量、体积或浓度。
这是演示店铺,请务下单付款,避免造成你的财物损失。
为了获得访问"阿拉丁铁蛋"实时聊天框的流畅支持体验,建议您使用Chrome浏览器或选择360浏览器极速模式(如何切换极速模式?),感谢您选择我们!

| 货号 (SKU) | 包装规格 | 是否现货 | 价格 | 数量 |
|---|---|---|---|---|
| N411994-1mg (试用装) 申请此免费试用装(?) 作为尊贵的客户,您每年可免费申请一次试用装产品,尽享探索与享受!
|
1mg |
现货  |
| |
| N411994-5mg |
5mg |
现货  |
| |
| N411994-10mg |
10mg |
现货  |
|
| 产品名称 | Nivolumab (anti-PD-1) |
|---|---|
| 别名 | 纳武单抗; Nivolumab (anti-PD-1); Recombinant Nivolumab Antibody; BMS-936558; ONO-4538; MDX-1106; Opdivo; Anti-PDCD1 / PD-1 / CD279 Reference Antibody (nivolumab); CD279 antibody; CD279 antigen antibody; HPD 1 antibody; HPD l antibody; HPD-1 antibody; HSLE1 a |
| 英文别名 | Nivolumab (anti-PD-1); Recombinant Nivolumab Antibody; BMS-936558; ONO-4538; MDX-1106; Opdivo; Anti-PDCD1 / PD-1 / CD279 Reference Antibody (nivolumab); CD279 antibody; CD279 antigen antibody; HPD 1 antibody; HPD l antibody; HPD-1 antibody; HSLE1 antibod |
| 规格或纯度 | Purity>95% (SDS-PAGE&SEC); Endotoxin Level<1.0EU/mg; Human IgG4SP; CHO; ELISA, FACS, Functional assay, Animal Model; Unconjugated |
| 特异性 | PDCD1/PD-1/CD279 |
| 应用 | ELISA,Functional Assay,Flow cytometry,Kinetics (BLI),Kinetics (SPR) |
| 反应种属 | Cynomolgus,Human |
| 抗体类型 | Primary antibody |
|---|---|
| Format | Whole IgG |
| 亚型 | Human IgG4SP |
| 轻链亚型 | Kappa |
| SDS-PAGE | 26.4 kDa (Light Chain) & 53.3 kDa (Heavy Chain), under reducing conditions; 178.6 kDa, under non-reducing conditions. |
| 纯化方法 | Protein A purified |
| 来源 | CHO supernatant |
| 物理外观 | Liquid |
| 储存缓冲液 | Supplied as a 0.22 μm filtered solution in 100mM Pro-Ac, 20mM Arg, pH 5.0 |
| 防腐剂 | No |
| 浓度 | Lot by Lot |
| 储存温度 | -80℃储存,避免反复冻融 |
| 运输条件 | 超低温冰袋运输 |
| 稳定性与储存 | Store at -80℃ for 18 months. Upon delivery aliquot. Avoid freeze/thaw cycle. |
| CAS编号和信息 | 946414-94-4 |
| IMGT/mAb-DB | 424 |
|---|---|
| Wikipedia | nivolumab |
| PubChem SID | 178103907 |
| Reactome Reaction | R-HSA-9679421 |
| Reactome Drug | R-ALL-9679411 |
| PEP | nivolumab |
输入批号以搜索COA:
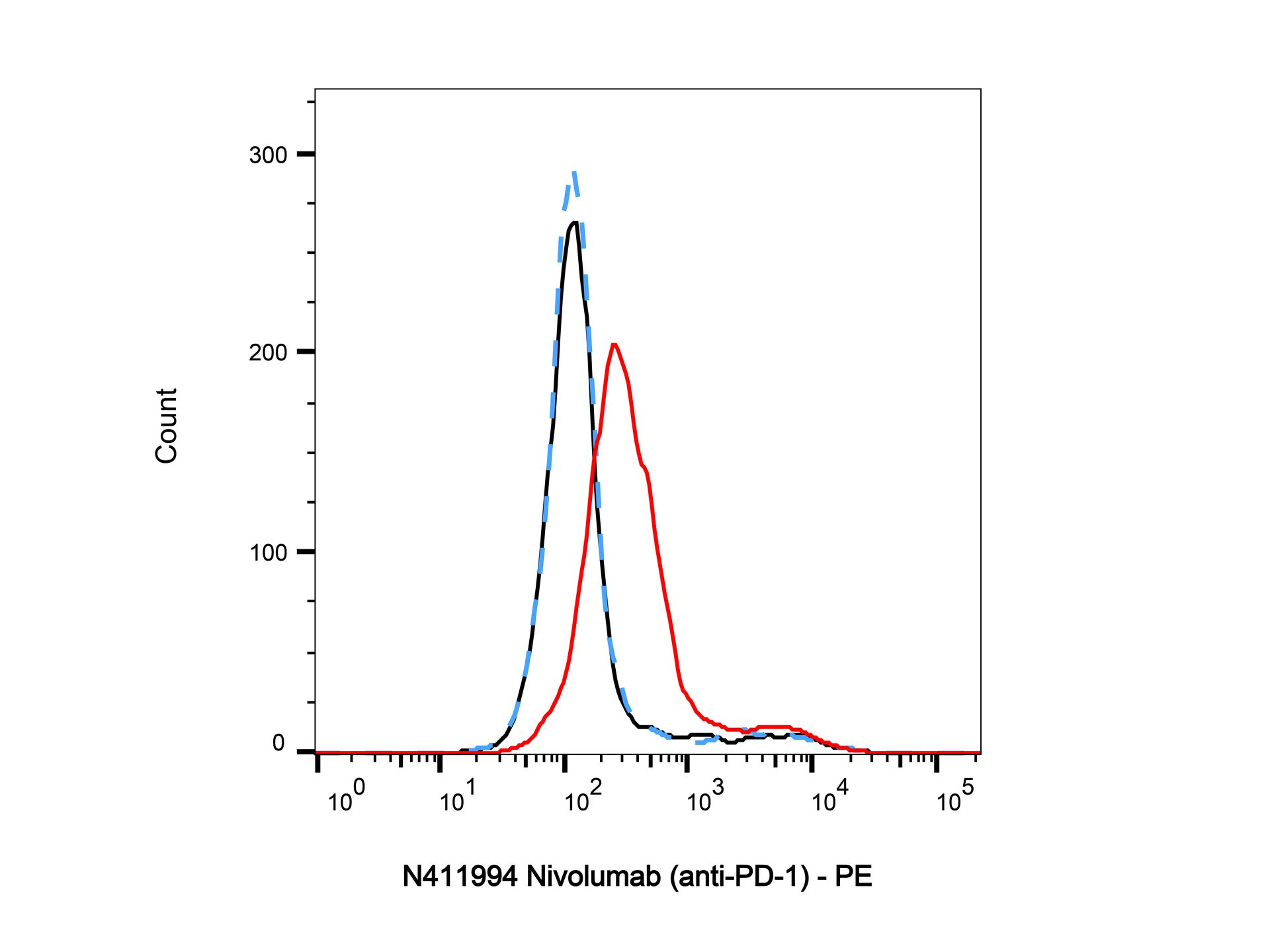
Nivolumab (anti-PD-1) (N411994) - Flow Cytometry
Flow Cytometry analysis of PHA-stimulated (3 days) human peripheral blood mononuclear lymphocytes labelling PD-1 (red) with Nivolumab (anti-PD-1) (N411994). Goat Anti-Human IgG (PE) (Ab175838) at a dilution of 1/1000 was used as the secondary antibody. Blue - Isotype control, human IgG (Ab170213). Black - Unlabelled control, cells without incubation with primary antibody.

Nivolumab (anti-PD-1) (N411994) - SEC
The purity of Nivolumab (anti-PD-1) (N411994) is more than 95% verified by HPLC.
| 1. Janakiram M, Abadi YM, Sparano JA, Zang X. (2012) T cell coinhibition and immunotherapy in human breast cancer.. Discov Med, 14 (77): (229-36). [PMID:23114578] |
| 2. Greaves P, Gribben JG. (2013) The role of B7 family molecules in hematologic malignancy.. Blood, 121 (5): (734-44). [PMID:23223433] |
| 3. Tang PA, Heng DY. (2013) Programmed death 1 pathway inhibition in metastatic renal cell cancer and prostate cancer.. Curr Oncol Rep, 15 (2): (98-104). [PMID:23263823] |
| 4. Hamid O, Carvajal RD. (2013) Anti-programmed death-1 and anti-programmed death-ligand 1 antibodies in cancer therapy.. Expert Opin Biol Ther, 13 (6): (847-61). [PMID:23421934] |
| 5. Intlekofer AM, Thompson CB. (2013) At the bench: preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy.. J Leukoc Biol, 94 (1): (25-39). [PMID:23625198] |
| 6. Callahan MK, Wolchok JD. (2013) At the bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy.. J Leukoc Biol, 94 (1): (41-53). [PMID:23667165] |
| 7. O'Sullivan Coyne G, Madan RA, Gulley JL. (2014) Nivolumab: promising survival signal coupled with limited toxicity raises expectations.. J Clin Oncol, 32 (10): (986-8). [PMID:24590655] |
| 8. Luke JJ, Ott PA. (2015) PD-1 pathway inhibitors: the next generation of immunotherapy for advanced melanoma.. Oncotarget, 6 (6): (3479-92). [PMID:25682878] |
| 9. Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS et al.. (2015) Nivolumab and ipilimumab versus ipilimumab in untreated melanoma.. N Engl J Med, 372 (21): (2006-17). [PMID:25891304] |
| 10. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P et al.. (2015) Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma.. N Engl J Med, 373 (1): (23-34). [PMID:26027431] |
| 11. Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER et al.. (2015) Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma.. N Engl J Med, 373 (19): (1803-13). [PMID:26406148] |
| 12. Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P, Porta C, George S et al.. (2018) Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma.. N Engl J Med, 378 (14): (1277-1290). [PMID:29562145] |
| 13. Pardoll DM. (2012) The blockade of immune checkpoints in cancer immunotherapy.. Nat Rev Cancer, 12 (4): (252-64). [PMID:22437870] |
| 14. Topalian SL, Drake CG, Pardoll DM. (2015) Immune checkpoint blockade: a common denominator approach to cancer therapy.. Cancer Cell, 27 (4): (450-61). [PMID:25858804] |